The invention:
The culmination of centuries of efforts to mimic the
brilliant colors displayed in nature in dyes that can be used in
many products.
The people behind the invention:
Sir William Henry Perkin (1838-1907), an English student in
Hofmann’s laboratory
René Bohn (1862-1922), a synthetic organic chemist
Karl Heumann (1850-1894), a German chemist who taught Bohn
Roland Scholl (1865-1945), a Swiss chemist who established the
correct structure of Bohn’s dye
August Wilhelm von Hofmann (1818-1892), an organic chemist
Synthesizing the Compounds of Life
From prehistoric times until the mid-nineteenth century, all dyes
were derived from natural sources, primarily plants. Among the
most lasting of these dyes were the red and blue dyes derived from
alizarin and indigo.
The process of making dyes took a great leap forward with the
advent of modern organic chemistry in the early years of the nineteenth
century. At the outset, this branch of chemistry, dealing with
the compounds of the element carbon and associated with living
matter, hardly existed, and synthesis of carbon compounds was not
attempted. Considerable data had accumulated showing that organic,
or living, matter was basically different from the compounds
of the nonliving mineral world. It was widely believed that although
one could work with various types of organic matter in
physical ways and even analyze their composition, they could be
produced only in a living organism.
Yet, in 1828, the German chemist Friedrich Wöhler found that it
was possible to synthesize the organic compound urea from mineral
compounds. As more chemists reported the successful preparation
of compounds previously isolated only from plants or animals,
the theory that organic compounds could be produced only in a living
organism faded.
One field ripe for exploration was that committed to exploiting the
uses of coal tar. Here, August Wilhelm von Hofmann was an active
worker. He and his students made careful studies of this complex
mixture. The high-quality stills they designed allowed for the isolation
of pure samples of important compounds for further study.
Of greater importance was the collection of able students Hofmann
attracted. Among them was Sir William Henry Perkin, who is regarded
as the founder of the dyestuffs industry. In 1856, Perkin undertook
the task of synthesizing quinine (a bitter crystalline alkaloid
used in medicine) from a nitrogen-containing coal tar material
called toluidine. Luck played a decisive role in the outcome of his
experiment. The sticky compound Perkin obtained contained no
quinine, so he decided to investigate the simpler related compound
aniline.Asmall amount of the impurity toluidine in his aniline gave
Perkin the first synthetic dye, Mauveine.
Searching for Structure
From this beginning, the great dye industries of Europe, particularly
Germany, grew. The trial-and-error methods gave way to more
systematic searches as the structural theory of organic chemistry
was formulated.
As the twentieth century began, great progress had been made,
and German firms dominated the industry. Badische Anilin- und
Soda-Fabrik (BASF) was incorporated at Ludwigshafen in 1865 and
undertook extensive explorations of both alizarin and indigo. A
chemist, René Bohn, had made important discoveries in 1888, which
helped the company recover lost ground in the alizarin field. In
1901, he undertook the synthesis of a dye he hoped would combine
the desirable attributes of both alizarin and indigo.
As so often happens in science, nothing like the expected occurred.
Bohn realized that the beautiful blue crystals that resulted
from his synthesis represented a far more important product. Not
only was this the first synthetic vat dye, Indanthrene, ever prepared,
but also, by studying the reaction at higher temperature, a useful
yellow dye, Flavanthrone, could be produced.
The term vat dye is used to describe a method of applying the dye,
but it also serves to characterize the structure of the dye, because all
currently useful vat dyes share a common unit. One fundamental
problem in dyeing relates to the extent to which the dye is watersoluble.
Abeautifully colored molecule that is easily soluble in water
might seem attractive given the ease with which it binds with the fiber;
however, this same solubility will lead to the dye’s rapid loss in
daily use.
Vat dyes are designed to solve this problem by producing molecules
that can be made water-soluble, but only during the dyeing or
vatting process. This involves altering the chemical structure of the
dye so that it retains its color throughout the life of the cloth.
By 1907, Roland Scholl had showed unambiguously that the
chemical structure proposed by Bohn for Indanthrene was correct,
and a major new area of theoretical and practical importance was
opened for organic chemists.
Impact
Bohn’s discovery led to the development of many new and useful
dyes. The list of patents issued in his name fills several pages in
Chemical Abstracts indexes.
The true importance of this work is to be found in a consideration
of all synthetic chemistry, which may perhaps be represented by
this particular event. More than two hundred dyes related to Indanthrene
are in commercial use. The colors represented by these substances
are a rainbow making nature’s finest hues available to all.
The dozen or so natural dyes have been synthesized into more than
seven thousand superior products through the creativity of the
chemist.
Despite these desirable outcomes, there is doubt whether there is
any real benefit to society from the development of new dyes. This
doubt is the result of having to deal with limited natural resources.
With so many urgent problems to be solved, scientists are not sure
whether to search for greater luxury. If the field of dye synthesis reveals
a single theme, however, it must be to expect the unexpected.
Time after time, the search for one goal has led to something quite
different—and useful.
William Henry Perkin
Born in England in 1838,William Henry Perkin saw a chemical
experiment for the first time when he was a small boy. He
found his calling there and then, much to the dismay of his father,
who wanted him to be a builder and architect like himself.
Perkin studied chemistry every chance he found as a teenager
and was only seventeen when he won an appointment as
the assistant to the German chemist August Wilhelm von Hofmann.
A year later, while trying to synthesize quinine at Hofmann’s
suggestion, Perkin discovered a deep purple dye—now
known as aniline purple or Mauveine, but popularly called
mauve. In 1857 he opened a small dyeworks by the Grand
Union Canal in West London, hoping to make his fortune by
manufacturing the dye.
He succeeded brilliantly. His ambitions were helped along
royally when Queen Victoria wore a silk gown dyed with Mauveine
to the Royal Exhibition of 1862. In 1869, he perfected a
method for producing another new dye, alizarin, which is red.
A wealthy man, he sold his business in 1874 when he was just
thirty-six years old and devoted himself to research, which included
isolation of the first synthetic perfume, coumarin, from
coal tar.
Perkin died in 1907, a year after receiving a knighthood, one
of his many awards and honors for starting the artificial dye industry.
His son William Henry Perkin, Jr. (1860-1927) also became
a well-known researcher in organic chemistry.
See also: Buna rubber; Color film; Neoprene.

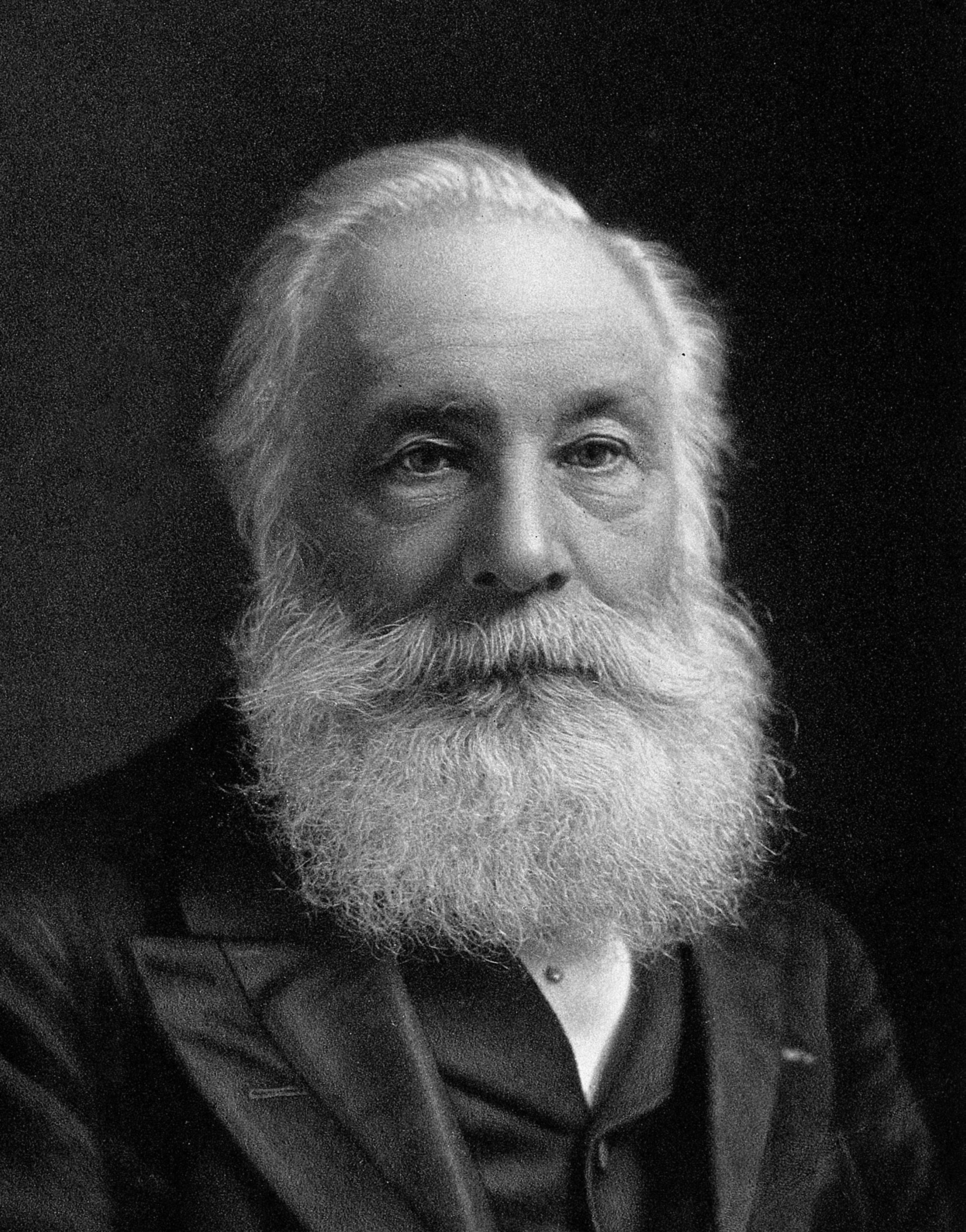
2 comments:
We make the custom synthesis process more efficient and cost effective while maintaining the highest standards of quality and reliability. FLAVANTHRONE
Harrah's Reno Casinos & Casinos - Mapyro
Find Harrah's Reno Casinos & Casinos in Reno, NV, United 수원 출장마사지 States - Mapyro® and 경산 출장샵 other nearby real money 파주 출장샵 casinos and 서귀포 출장안마 other places to stay in and play in 김천 출장안마
Post a Comment